3 December 2019
New study finds the mix that makes Titan’s lakes spew nitrogen bubbles
Posted by larryohanlon
By Erin I. Garcia de Jesus
New research explains how bubbles erupt in frigid hydrocarbon lakes on Saturn’s largest moon Titan, potentially creating fizz intense enough to form geologic features on the moon.
Titan is covered in hydrocarbon lakes made up of methane and ethane. Scientists have noticed bright spots in these lakes, which appeared in some pictures from NASA’s Cassini spacecraft and mysteriously vanished in others. They later theorized these “magic islands” might be outbursts of nitrogen bubbles.

An artist’s depiction of Winnipeg Lacus, a hydrocarbon lake close to Titan’s north pole. Credit: NASA/JPL-Caltech
In the new study published in AGU’s journal Geophysical Research Letters, researchers simulated Titan’s lakes in a pressurized chamber. They found the right combination of methane, ethane and nitrogen crucial for bubbles to form.
Under conditions most like those on Titan, the researchers found ethane had to flow into pools of methane to produce vigorous bubbles. It is possible these bubble outbreaks are strong enough to shape river deltas in bodies of liquid on the moon, according to the new research.
Explaining how bubbles form in Titan’s lakes now allows scientists to begin probing fundamental questions about how liquids behave on the moon. Of all the bodies in our solar system, few are more Earth-like than Titan, and it is one of the few places scientists think might have conditions necessary for extraterrestrial life.
The results also hint at scenarios an exploratory submarine might face in Titan’s lakes, if the spacecraft were to give off heat and potentially spark an explosion of bubbles.
“The more we learn about Titan, the more we learn that we can’t ignore the lakes,” said Kendra Farnsworth, a planetary scientist at the University of Arkansas in Fayetteville and lead author of the new study. “And we find fun things like bubbles. Maybe a little bit more violent than we’d expected, but definitely fun to watch.”
Making bubbles
Titan is the only moon in our solar system to have an atmosphere. Its air is composed mainly of nitrogen—an element that also forms the bulk of Earth’s atmosphere—and hydrocarbons, which form a thick, hazy layer obscuring many of the features across its surface.
Titan’s clouds deliver hydrocarbon rain in the form of methane and ethane. On Earth, methane is a gas used for heating, cooking and electricity, while ethane gas is a precursor for polyethylene plastic.
Temperatures on Titan, however, are cold enough for these compounds to be liquids. There, hydrocarbons cycle through the atmosphere much like water does on Earth. Liquid methane and ethane lakes sprinkle Titan’s surface—making it the only other body in our solar system besides Earth to host stable fluids.
Previous work found nitrogen gas from Titan’s atmosphere could readily dissolve into cold pools with high concentrations of methane—like when carbon dioxide dissolves into soda. Upon heating, the liquid released nitrogen gas in the form of fizzing bubbles.
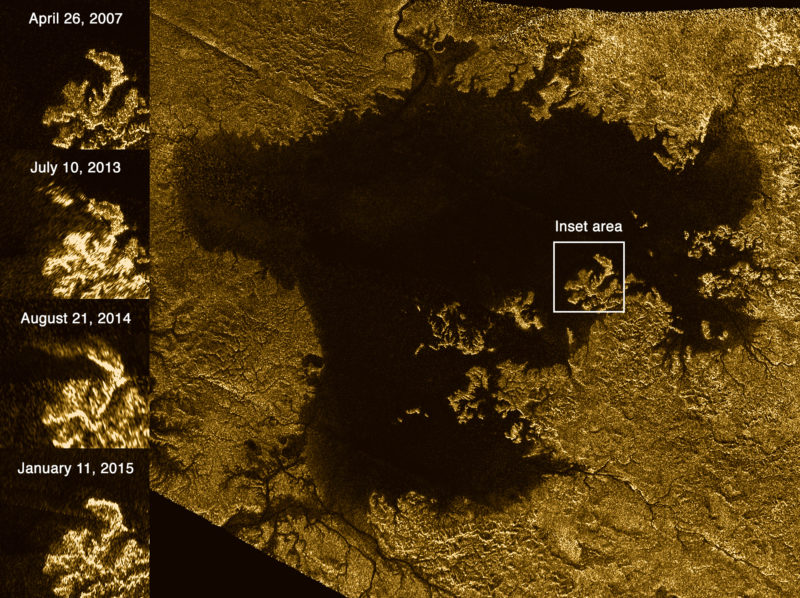
These images from the Radar instrument aboard NASA’s Cassini spacecraft show the evolution of a transient feature, informally known as a “magic island”, in the large hydrocarbon sea named Ligeia Mare on Saturn’s moon Titan. Credit: NASA/JPL-Caltech/ASI/Cornell
But these earlier experiments didn’t fully mimic the natural environment on Titan. They also didn’t explain what conditions could make the lakes foam, although researchers suspected it happens during heavy rainfall or when a stream flows into a lake.
“Titan’s lakes have very interesting dynamics,” Farnsworth said. “They’re not just static bodies of liquid.”
Titan, but on Earth
To determine how ethane, methane and nitrogen might burst into bubbles on Titan, Farnsworth and her colleagues conducted experiments in a six-foot tall, pressurized chamber simulating conditions on the moon. Inside, they set the atmospheric pressure to 1.5-bar—which is 1.5 times higher than Earth’s at sea level—and temperatures ranged from a brisk 83 degrees Kelvin (-190 Celsius or -310 Fahrenheit) to a balmy 94 degrees Kelvin (-179 Celsius or -290 Fahrenheit).
The researchers allowed one liquid to flow into a sample dish containing a pool of the other. The researchers then cooled the inside of the chamber until it was either above or below 86 degrees Kelvin (-187 Celsius or -305 Fahrenheit) to let nitrogen dissolve. In one set of experiments, ethane flowed into ponds of methane. In another, methane flowed into ethane. The team then gradually warmed up the chamber and waited for bubbles to erupt.
Two scenarios resulted in bubbles. At temperatures below 86 degrees Kelvin, ethane layered on top of nitrogen-rich methane, no matter what order they were poured into the petri dish. As the temperature warmed, the methane underneath began to foam and when the layers dissolved, bubbles reached the surface.
If the chamber was above 86 degrees Kelvin when the researchers added the liquids, methane flowing into ethane didn’t yield any foam. Only ethane flowing into methane pools produced bubbles—and did so forcefully.
“The most surprising thing was how violent the explosions were,” Farnsworth said. During one experiment, the outburst of bubbles was so strong, it affected the equipment. “All of a sudden, I look over and the bubbles literally blew up and hit my camera,” she recalled.
Fun fizz
The new results suggest changes in both temperature and composition are crucial for bubbles to form in Titan’s lakes.
Bubbles erupt when flowing ethane mixes with methane supersaturated with nitrogen—meaning the methane contains more dissolved nitrogen than normal conditions allow. When the methane warms, it can hold less dissolved nitrogen, which escapes as gas. The mixture must also have between 40 and 95 percent methane to make bubbles, according to the study.
It’s almost like making rock candy. When boiling water is supersaturated with sugar—or contains more sugar than can normally be dissolved—crystals will form on a piece of sugar-coated wood as the liquid cools.
Experiments at warmer temperatures most likely mimic what is happening on Titan’s surface because the moon’s coldest temperatures dip down to only 89 degrees Kelvin (-184 Celsius or-299 Fahrenheit), Farnsworth said.
“[The new study] is a nice piece of work that adds to what we are learning about Titan’s lakes and emphasizes how important laboratory work is,” said Michael Malaska, a planetary scientist at NASA’s Jet Propulsion Laboratory in California who was not involved in the work. “It’s like ‘welcome to the weird’ and is a different way of thinking about something that’s not water.”
Erin I. Garcia de Jesus is a science writing intern at AGU. Follow her on Twitter @viruswhiz


 GeoSpace is a blog on Earth and space science, managed by AGU’s Public Information staff. The blog features posts by AGU writers and guest contributors on all sorts of relevant science topics, but with a focus on new research and geo and space sciences-related stories that are currently in the news.
GeoSpace is a blog on Earth and space science, managed by AGU’s Public Information staff. The blog features posts by AGU writers and guest contributors on all sorts of relevant science topics, but with a focus on new research and geo and space sciences-related stories that are currently in the news.