3 November 2016
Rare molecule on Venus could shed light on planet’s weather
Posted by dgaristo

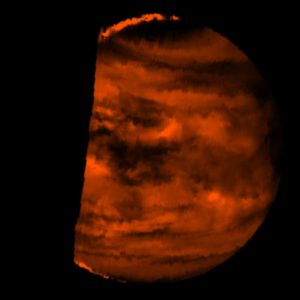
A composite image of Venus’ northern hemisphere. Credit: Solar System Visualization Project and the Magellan Science team
New study finds uncommon sulfur compound could be absorbing ultraviolet rays in Venus’s atmosphere
By Dan Garisto
Scientists’ keen detective work may have solved one of Venus’s oldest secrets: why the planet’s atmosphere absorbs a specific frequency of ultraviolet light. The new findings could help scientists better understand Venus’s thick atmosphere and its heat-trapping clouds, according to the study’s authors.
Scientists first noticed Venus’s thick, cloudy atmosphere absorbing near UV light coming from the sun in the 1970s, but at the time, they were not able to determine which molecule was responsible for absorbing the rays.
The new study suggests the culprit is disulfur dioxide, a rare gas composed of two sulfur and two oxygen atoms which does not exist on Earth. It could be absorbing much of the ultraviolet (UV) light entering Venus’s atmosphere, similar to the way Earth’s ozone layer protects our planet from UV rays, which cannot be seen by the human eye but can cause sunburns.
Using advanced modeling, the scientists showed the unusual molecule can form on Venus and is likely an important component of the planet’s thick clouds.
If their hypothesis is correct, the findings could help scientists better understand Venus’s sulfur cycle, a key component of weather on Venus, according to Henrik Kjaergaard, a professor of chemistry at the University of Copenhagen and co-author of the study published in Geophysical Research Letters, a journal of the American Geophysical Union.
“It’s cool to explain this missing absorber,” he said. “So, as a scientist, that’s really fun. But I think in the bigger picture on the Venus atmosphere it means that the atmospheric models that are currently out there need to include these mechanisms and new compounds.”
Solving the mystery of the missing UV absorber may also help explain Venus’s ability to trap heat. Venus is covered by a thick, cloudy atmosphere many times denser than Earth’s. The thick clouds trap heat to keep the surface a toasty 470 degrees Celsius (870 degrees Fahrenheit) — making Venus the hottest planet in our solar system.
The presence of large amounts of disulfur dioxide would have an important effect on Venus’s atmosphere, according to Yuk Yung, a planetary researcher at California Institute of Technology in Pasadena, who was not involved with the new study. In addition to its role in the sulfur cycle, the compound would likely be a major factor in Venus’s soaring temperatures. Disulfur dioxide would trap heat from the sun through its absorption of UV rays, he said.
Out of this world
To test which sulfur compound the mystery absorber could be, the scientists “built” molecules in a computer simulation by solving quantum chemical equations for their constituent atoms.
Using the information from the equations, scientists could calculate each molecule’s energy levels. Molecules with different energy levels absorb light at different wavelengths, so Kjaergaard and his co-authors screened the compounds by energy level to find one that would absorb UV light at the same wavelengths — 320 to 400 nanometers — that Venus’s atmosphere absorbs UV light.
Sulfur monoxide and sulfur dioxide exist in high enough concentrations on Venus to be candidates for the mystery absorber, but neither compound absorbs UV light between 320 and 400 nanometers, so the scientists ruled them out. Although disulfur dioxide was not known to exist on Venus, scientists had theorized it could form on the hot planet under certain conditions, Kjaergaard said.
Disulfur dioxide molecules take on several different configurations, called isomers. Using their quantum chemical model, the researchers predicted which disulfur dioxide isomers would form under the theorized conditions. They found two disulfur dioxide isomers that fit the bill for absorbing UV light.
Finally, the researchers calculated how fast disulfur dioxide isomers would form and break down in the Venusian atmosphere to estimate how abundant the molecules were. The numbers proved startlingly high: In the top cloud layer, disulfur dioxide could be as abundant as sulfur monoxide or sulfur dioxide — the most plentiful species of sulfur in the Venusian atmosphere, according to Kjaergaard.
To confirm the UV absorber is disulfur dioxide, the scientific community would need to send a spacecraft to Venus to measure it directly, according to Yung. In the meantime, Kjaergaard and his team will attempt to create disulfur dioxide in their lab to experimentally measure properties like its heat trapping potential.
—Dan Garisto is a science writing intern at the American Geophysical Union.










 GeoSpace is a blog on Earth and space science, managed by AGU’s Public Information staff. The blog features posts by AGU writers and guest contributors on all sorts of relevant science topics, but with a focus on new research and geo and space sciences-related stories that are currently in the news.
GeoSpace is a blog on Earth and space science, managed by AGU’s Public Information staff. The blog features posts by AGU writers and guest contributors on all sorts of relevant science topics, but with a focus on new research and geo and space sciences-related stories that are currently in the news.