16 March 2014
Severe Travel Restrictions Imposed In Paris as Smog Worsens
Posted by Dan Satterfield
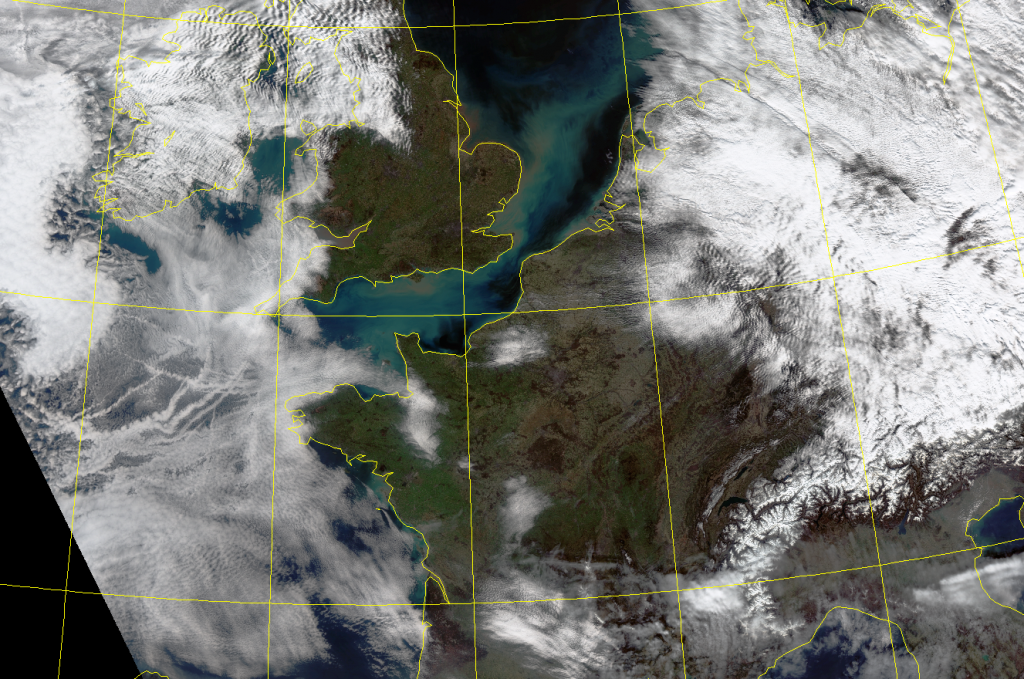
Clear skies and light winds in the low levels of the atmosphere have caused a strong inversion to develop over much of France. This has led to very high air pollution levels. Image from SUOMI VIRRS sensor. Courtesy Uni. of Dundee in Scotland. Click for LARGE version.
Paris just cut the vehicles on Monday’s roads by half in an effort to improve the air quality over the Capital. It also has made all mass transit free, and the BBC has further details here. Motorcycles (most of who have rather dirty engines) are also included.
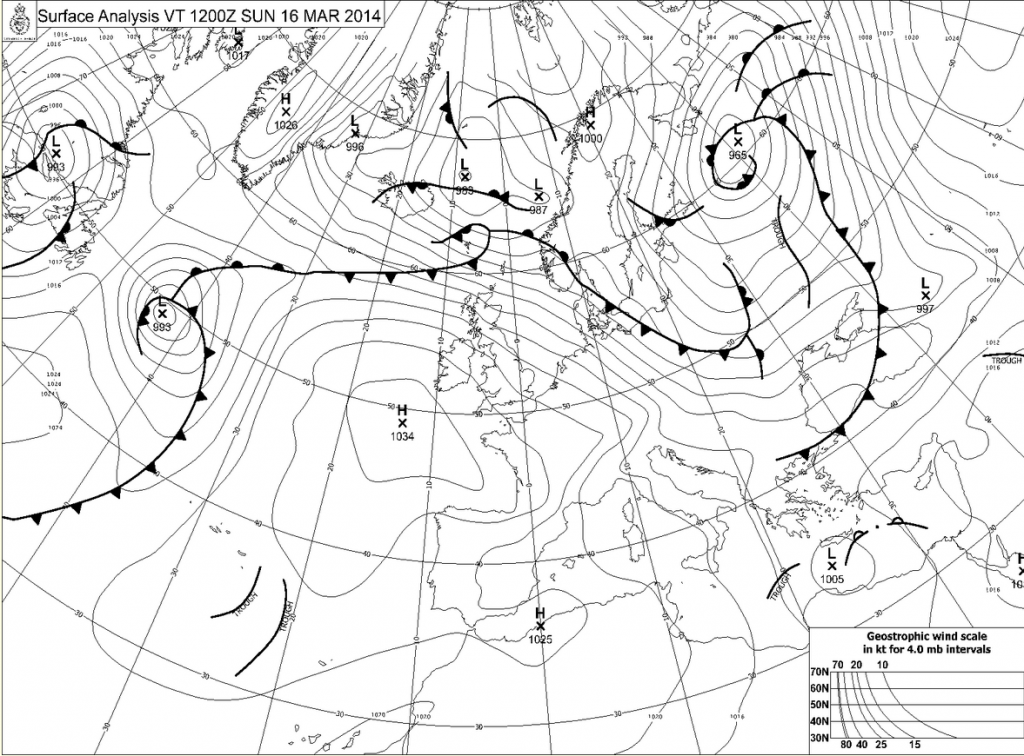
Map from the UK Met office this morning showing a large high pressure ridge over France. Click for much larger version.
The reason for the bad smog is a temperature inversion, and you have likely heard this before, but I want to explain WHY a temperature inversion causes poor air quality. The inversion developed because the ground is still cool, and skies are clear under a high pressure center. This high pressure has been over the area for days now and there is also high pressure aloft over the area and this is caused by warmer air aloft as well.
So we have a situation where the air near the ground is quite cool (the nights are still long and at that latitude the sun angle is still low.) and the air aloft is warmer with light winds.
The image below is the “RAOB sounding” taken by weather balloon at 1 PM France time (12 GMT). It shows temperature, dew-point, and winds from near the surface to around 250 millibars which is around 15 kilometers above the surface. As air rises, it will cool at a rate called the dry adiabatic lapse rate (More on lapse rates here).This is approx. 10°C/1000 meters. You can use the chart to see what temperature a bubble of sir would be if you lifted it from the ground to say 3 km (around 800 millibars).
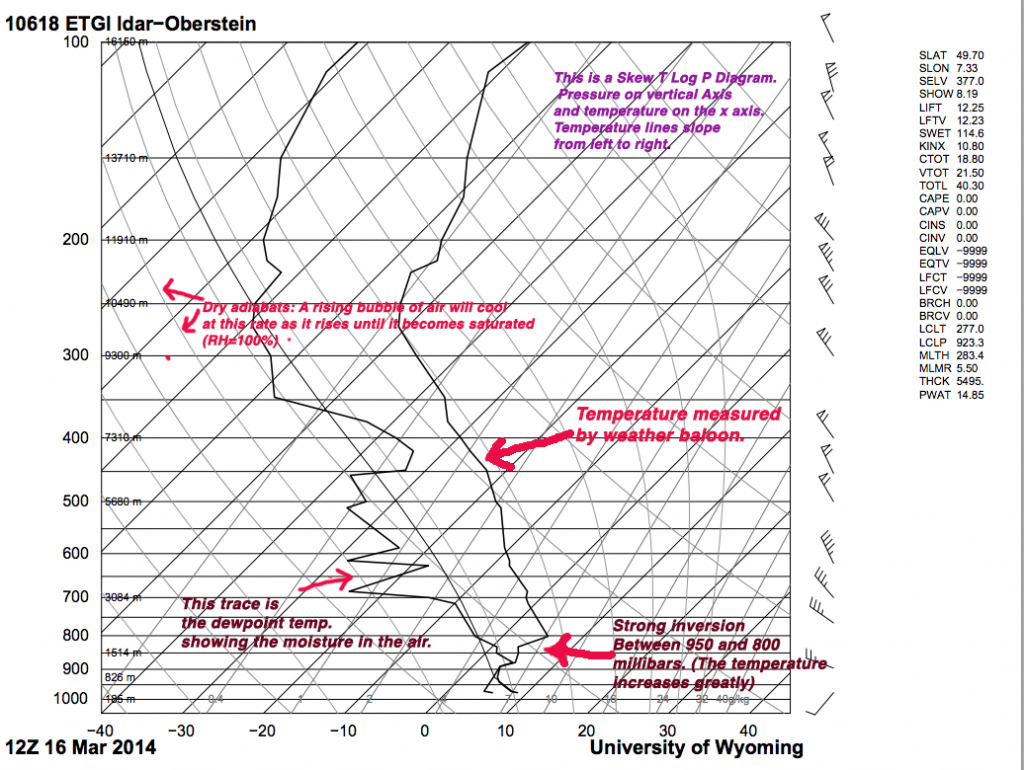
You will need to click the image to see it in full resolution. Sounding was from today and the balloon launch was at Oberstein in Germany. It’s about halfway between Reim in France, and Frankfurt and a good representative of the atmosphere over France and Luxembourg today.
If you take the air near the ground (which is about 9°C) and lift it up along the dry adiabat, you will see that it cools rapidly and is to the left of the air temperature on the sounding. A bubble of air rising up from the surface will find itself suddenly colder than the air around it, and will sink back down until it is about the same temperature again. What could cause the bubble of air to rise in the first place? Topographic features for one, but the Earth is heated from below, and the sun will warm the ground and the ground will warm the air with it, and a bubble of sun heated air will rise when it gets warmer than the air around it. The air at 800 millibars will change little between day and night however, so that rising bubble of air from near the ground will still cool rapidly and be colder than the air around it.
This fact causes the air to be trapped in a narrow layer near the ground, and all of the industrial emissions are trapped with it. Sunlight, being a key ingredient in smog is abundant as well with the clear skies under the high pressure cell. See if you can figure out what temperature at the surface would allow the air to escape??
Hint: Follow the dry adiabat from the top of the inversion to the surface (That’s how meteorologists do it!).


 Dan Satterfield has worked as an on air meteorologist for 32 years in Oklahoma, Florida and Alabama. Forecasting weather is Dan's job, but all of Earth Science is his passion. This journal is where Dan writes about things he has too little time for on air. Dan blogs about peer-reviewed Earth science for Junior High level audiences and up.
Dan Satterfield has worked as an on air meteorologist for 32 years in Oklahoma, Florida and Alabama. Forecasting weather is Dan's job, but all of Earth Science is his passion. This journal is where Dan writes about things he has too little time for on air. Dan blogs about peer-reviewed Earth science for Junior High level audiences and up.