10 June 2010
Graph beauty: T vs. viscosity for lavas
Posted by Callan Bentley
I spent the day lazily reading the igneous petrology chapters of Petrology by Blatt, Tracy, and Owens (third edition, 2006). Last time I read it, I didn’t get all that much from the igneous section, but this time around that’s the thing that motivated me to delve into it again. I don’t remember enough about igneous petrology from my school days, and while I have a little breathing room this summer, it seemed to me that I could bone up on it a bit.
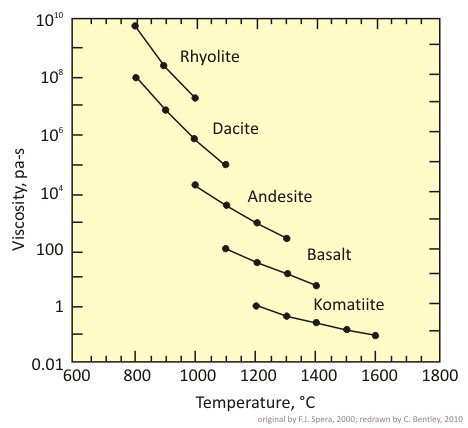
One thing that caught my eye this afternoon was Figure 4-15, on page 78. I have redrawn it for you here:
I love beautiful graphs like this. It compares viscosity (resistance to flow, as measured in pascal-seconds; each pa-s is the same as 10 poise) to temperature (as measured in degrees Celsius). Five different compositions of lava are plotted: komatiite, basalt, andesite, dacite, and rhyolite.
First off, you’re no doubt struck by the inverse relationship between temperature and viscosity. The hotter the lava is, the less viscous it is (more runny; easier to flow).
Second, higher-silica-content lavas (rhyolites, dacites) are much more viscous than lower-silica content lavas (basalts, komatiites). Silica (and, to a lesser extent, alumina) form polymer-like chains. The more silica there is (up to 75 wt% in some rhyolites), the more of these sticky, web-like chains can form. This is why you can see lava dripping off the molten basalt in this video, but the molten granite clings to its source rock. Water actually interrupts the formation of these silica polymers, and thus lowers viscosity when it is present.
I’m also struck, looking at this graph, of the difference in temperatures plotted from left to right. This corresponds with observed lava eruption temperatures of different compositions. Low-silica lavas erupt at high temperatures, as they are chock full of high-crystallization-temperature mineral components. (They wouldn’t erupt at all at lower temperatures, because they would be solid.) High-silica lavas erupt at relatively low temperatures, as the components they contain will crystallize into minerals like quartz at relatively low temperatures.
Ironically, though we might think “high temperatures = more dangerous,” the opposite is true. Low-silica lavas tend to erupt effusively, with relatively little risk for human life. You can outrun a lava flow — with rare exceptions, even low viscosity lavas still only flow a few hundred meters per hour. The lower-temperature lavas, on the other hand, are the ones to worry about — because they’re viscous. This “stickiness” means they tend to clog up magmatic plumbing and allow greater pressures to build up. Couple this idea with the tendency of high-silica lavas to also be rich in dissolved gases, and you get a much more explosive style of eruption.
________________________________________________
Reference
Spera, F.J. (2000) “Physical properties of magma,” in Encyclopedia of Volcanoes, H. Siggurdsson, ed. San Diego CA: Academic Press. {Fig. 4}


 Callan Bentley is Associate Professor of Geology at Piedmont Virginia Community College in Charlottesville, Virginia. He is a Fellow of the Geological Society of America. For his work on this blog, the National Association of Geoscience Teachers recognized him with the James Shea Award. He has also won the Outstanding Faculty Award from the State Council on Higher Education in Virginia, and the Biggs Award for Excellence in Geoscience Teaching from the Geoscience Education Division of the Geological Society of America. In previous years, Callan served as a contributing editor at EARTH magazine, President of the Geological Society of Washington and President the Geo2YC division of NAGT.
Callan Bentley is Associate Professor of Geology at Piedmont Virginia Community College in Charlottesville, Virginia. He is a Fellow of the Geological Society of America. For his work on this blog, the National Association of Geoscience Teachers recognized him with the James Shea Award. He has also won the Outstanding Faculty Award from the State Council on Higher Education in Virginia, and the Biggs Award for Excellence in Geoscience Teaching from the Geoscience Education Division of the Geological Society of America. In previous years, Callan served as a contributing editor at EARTH magazine, President of the Geological Society of Washington and President the Geo2YC division of NAGT.
Reminds me of slag from a blast furnace. Interesting read.