This will be the new home of my blog Georneys. I’ll be moving all of my posts here from the AGU blogosphere – and starting some new blogging in 2024. Stay tuned!
Georneys is Moving – and Where to Find Me

AGU has announced that it will be sunsetting the AGU Blogosphere. So, I am here to say goodbye – and to thank AGU for years of hosting my blog.
I started blogging for the AGU Blogosphere in 2011, when I was in graduate school. I’ve enjoyed my time blogging here over the years, particularly my “What to Buy a Geologist for Christmas” and my “Geology Word of the Week” posts. In recent years, as I’ve moved countries (twice!), gotten married, become a mother, and had a busy career in geology and climate change management, my blogging has become more intermittent. I was just starting to gain momentum with my blogging again last year when I received an email that the blogosphere would be shutting down. I didn’t have the heart, for a few months, to put up new posts. However, I plan to migrate all of my posts over to my new blog site – evelyngeorneys.com. I do hope that I can revive the blog there, even if I only post a couple of times a month.
In the meantime, I have started up an Instagram page @evelyn_georneys where I am sharing a geology picture (with a little geology information) every day. I shared my first daily picture there on January 1st – and today I’m up to my 50th picture. Sometimes I share a picture of a rock or mineral that I saw that day. Other times I share a picture from my 20+ years of geological adventures.

In addition to following me on my new blog site and on Instagram, you can also connect with me on LinkedIn.
Last but certainly not least, I want to announce that I published my first book Rocks & Minerals: An Illustrated Field Guide in December with Cider Mill Press (an imprint of HarperCollins). In this book, I describe 20 common rocks and 5 rare rocks and 20 common minerals and 5 rare minerals, including elkinstantonite named after the fabulous Lindy Elkins-Tanton. The book is not intended to be a comprehensive guide but rather a fun tour through the world of geology. I have included a fun fact about each rock and mineral (well, I think they’re fun, anyway!). The rock and mineral profiles are accompanied by beautiful illustrations by the very talented Vlad Stankovic. If you would like a copy, you can find it on Amazon or buy it from your local bookseller. I hope that you enjoy my book.

Without further ado, I must say farewell. Hope to see some of you on Instagram and elsewhere!
Geology Word of the Week: Q is for Quartzite

def. Quartzite:
A metamorphic rock that consists primarily of interlocking grains of the mineral quartz. Quartzite forms when quartz-rich sandstone undergoes metamorphism.
The term “quartzite” is an example of a geologic term that can be a little confusing. Most of the time, geologists use the term “quartzite” to refer to a metamorphic rock, which is the definition that I have provided above. Sometimes, geologists also use the term “quartzite” to refer to a sandstone that has quartz grains that are very well cemented together, but which has not been transformed in to a metamorphic rock. In my experience as a geologist, this second usage is much less common. I think it was more commonly used in the past. To distinguish between different types of quartzite, geologists can use the terms “metaquartzite” to refer to the metamorphic variety and the term “orthoquartzite” to refer to the sedimentary variety. For the rest of this post, I’ll focus on metaquartzite, which I will just call “quartzite”.
Quartzite is a beautiful metamorphic rock that, at first glance, sometimes looks like sandstone. Quartzite can even retain the original cross-bedding that can be seen in many sandstone rocks, for example as shown in the pictures of quartzite outcrops from Minas Gerais, Brazil, shown in this post.

However, the quartz grains in a quartzite look different to those in a sandstone – they have recrystallized so that that are more or less equal in size. Compared to the original quartz grains that were in the sandstone, quartz grains in quartzite are larger and more polygonal in shape. The grains are tightly interlocked. A characteristic of quartzite is that when it breaks, it will break through grains, not just along grain boundaries.
Here’s a closer view of a quartzite hand sample:


Quartzite is a very hard rock that is highly resistant to erosion. Thus, quartzite can often be found on ridges and on the tops of hills. From a distance, quartzite may look like sandstone – so you may need to look at it close up in order to identify it as quartzite. Quartzite is most commonly white or gray in color, but can also be other colors, such as tan, pink, and yellow.
That’s all for this week’s post – stay tuned for next week!
Geology Word of the Week: P is for Pahoehoe

def. Pahoehoe:
A type of lava with a smooth or undulating surface. Pahoehoe lava flows often resemble coils of rope and are most commonly found in basalt. Pahoehoe is Hawaiian word that is pronounced “pah-hoy-hoy”.
Pahoehoe is a term used to describe a lava flow that has a smooth or undulating surface. Many pahoehoe flows resemble coils of rope or, in some cases, messy piles of rope. Pahoehoe is mostly commonly found in basalt.
Pahoehoe forms when lava flows slowly and a thin crust of cooled material can form on top of the hot lava, preventing heat from escaping and allowing the lava to retain its fluidity (or to retain its low viscosity, to use a more formal geologic term). Lava that flows slowly and cools slowly will form volcanic rocks with smooth or undulating morphologies and, often, will form volcanic rocks that resemble coils or piles of rope.
When lava flows more quickly, the surface crust tends to break and the lava loses heat more quickly. The lava becomes less fluid (or more viscous, since cooler lava is more viscous), which makes the volcanic rock have a blocky, rough morphology. A lava flow with a blocky, rough morphology is known as a’a (pronounced “ah-ah”), another Hawaiian word.
Both pahoehoe and a’a lava flows can be produced at the same volcano. For example, pahoehoe lava flows may form when the lava flows slowly across a gentle slope. However, if the slope becomes steeper and lava starts to flow more quickly the morphology of the lava flow can change from pahoehoe to a’a.
There are many great places to see pahoehoe and a’a lava flows. For example, you can see these types of lava flows in basaltic rocks on the volcanic islands of Hawaii and Iceland. A few years ago, my husband and I went on vacation to the volcanic island of Reunion in the Indian Ocean. We had and incredible trip and saw many amazing lava flows. The pictures of pahoehoe and a’a lava flows in this post were taken during that vacation.
Here are some more pictures of pahoehoe lava flows:




And here are some pictures of a’a lava flows:



That’s all for this week’s word – stay tuned for next week!
Geology Word of the Week: O is for Outcrop

I attempted to revive the Geology Word of the Week earlier this year, but a few busy projects at work and also writing my first book derailed my plans. Let’s try again! I have a Geology Alphabet to finish. I’ll use some photos from my trip to Scotland earlier this year to illustrate this post. Without further ado, here’s the next word…
def. Outcrop:
An exposure of rock formation at the Earth’s surface. It is a place where rocks crop out, hence the name.
Outcrops are very important for geologists. They are places where rock formations are exposed at the surface of the Earth. In many landscapes, rock formations are hidden underneath soil and vegetation. Even in the desert, rocks can be hidden underneath sand. Thus, in may places it is challenging for geologists to understand what rock formations lie underneath our feet. Outcrops are key locations where geologists can directly observe, sample, and measure rock formations.
Geologic maps are often made by collecting data from a number of outcrops and then interpreting the geology in between, which is often hidden underneath “cover”, a term that can be used to refer to soil, sand, vegetation, and so on that are covering up the rocks underneath. When working on a geologic map, geologists often take structural measurements of outcropping rocks, which provides key information about the directions in which rocks are titled, folded, and so on. The structural measurements help geologists better piece together the geology that is located underneath the cover.
Note that the term “cover” can also refer to other rock formations. Sometimes, geologists look for exposures of particular rock formations (for example, one which contains a rare ore), which may be hidden by cover that consists of other rocks (rather than soil, etc.).
Importantly, outcrops should consist of in situ rocks. That is, they should consist of rocks that are intact and which not have been transported by weathering processes. Sometimes, it can be difficult to tell at first glance if an exposed rock is an outcrop or if it is something that geologists call a “float”. A “float” is a rock or rock formation fragment that has been moved from its original location, for example by breaking off (through physical weathering) and falling down the side of a hill or, as another example, by water transport in a river or stream. In particular, it is easy to mistake large float rocks for outcrops, so it is important for geologists to take a close look and make sure they are inspecting an outcrop rather than a float. This is especially important for structural measurements, which will be incorrect for a piece of float compared to an actual outcrop.
Outcrops can be found in many locations. One great place to look for an outcrop is a cliff. Often, rock formations are exposed at cliffs. Thus, they are great places to see rocks that may be under cover elsewhere in the landscape. However, be careful when looking at rocks at a cliff. Rockfalls are common at cliffs, so you need to be cautious and take a look around for loose rocks that could fall. It may be wise to wear a helmet when studying geology near a cliff. If you are at the top of the cliff, you also need to be very careful not to fall down the cliff, however tempting it may be to get a better look at the rocks over the side!

Cliff outcrops look similar to another place where geologists often look for rocks: a roadcut, which is an artificial exposure of a rock formation that is created when rocks are blasted away to create a path for a road. Roadcuts are great places to study geology, although they are not true outcrops. It is important to remember that roadcuts will have artificial shapes and can have blasting and drilling marks, so you need to apply a little caution when interpreting geology at roadcuts. Of course, you also need to be careful not to be hit by cars when studying rocks at a roadcut!
Another good place to hunt for outcropping rocks is in the bed of a stream or river, where erosion by water can expose rocks underneath soil and vegetation. Erosion by ocean waves can also create outcrops of rock close to the sea.


Outcrops are also often found at the tops of hills or mountains, where resistant rocks can form rounded or jagged peaks. It can be more difficult for soil and vegetation to coat the tops of hills or mountains, due to factors such as steep terrain, wind, and altitude.

Outcrops are great places to get a close-up look at various geological features. For example, on one of the Orkney Island outcrops I was able to observe some neat mud cracks:

However, geologists do need to be careful when using outcrops to interpret what rocks are present under our feet. Harder, more durable rocks that are more resistant to erosion are more likely to be exposed in outcrop, while softer rocks may be more difficult to find in outcrop. Similarly, certain rock types, such as basalt and limestone, are more likely to form cliffs than other types of rocks. Thus, it is important not to overinterpret the abundance of rocks exposed in outcrop. To better understand the geology that lies under our feet, geologists must also use other methods, such as drilling to bring up cores of rock and deployment of geophysical instruments, together with study of rocks in outcrops.
That’s all for this week’s geology word. Stay tuned for next week!
Geology Word of the Week: N is for Notebook

def. Notebook:
A small book for recording notes, such as geological data. Since geologists often have to record notes in rainy (and sometimes snowy!) conditions, they prefer to use notebooks made with special paper that is water repellent and can be written on with a pencil or special pen even when there is precipitation.
When I was a geology student, I quickly learned that conducting geological fieldwork with a normal notebook is only possible on sunny days. When there is rain, snow, or sleet, it is nearly impossible to take notes in a notebook with normal paper. Fortunately, I soon realized that there are a few companies that produce special notebooks made with marvelous paper that is essentially waterproof. This paper is thick and feels a bit waxy. You cannot write on it with a normal pen, but you can write on it with a pencil or a special type of pen. The paper almost seems magical – it does not become weak or tear when it becomes wet. I have managed to successfully write in these special notebooks during the most horrible weather conditions, such as during heavy rain or sleet. I have even accidentally dropped my entire notebook in a stream and picked up my completely soaked notebook to find that my notes were in perfect condition. I’m not quite sure how these magical notebooks are made, but I do know that I use them whenever I conduct fieldwork of any sort.
There are a few companies that make waterproof notebooks. However, my favorite brand is Rite in the Rain. In particular, I like to use their Geological Field Book product (and, no, I am not being paid to sponsor this product – it’s just my favorite). This notebook has great lined pages for filling in geological information and making geological sketches. In the back, it has several pages of useful geological information for reference. The book also has a small plastic ruler that is located in a little pocket on the inside of the back cover. The ruler is perfect to use as a scale in geological photographs.
When I only need to make a few short notes in the field, I sometimes use a smaller, simpler notebook. For example, this little spiral notebook can fit in the pocket of my field shirt. I generally use a pencil for taking my field notes. However, I also sometimes use one of the special pens that are made for writing on the water repellent paper.
Here are a few more pictures of my favorite geological notebook:




Geology Word of the Week: M is for Mica

def. Mica:
A term used to describe a group of minerals that form in flat layers (or sheets) and have a vitreous or pearly luster (they are shiny!). Micas are phyllosilicate minerals, also known as “sheet silicate” minerals. Micas are common rock-forming minerals, although some varieties are harder to find than others. Micas come in many different colors. Common mica minerals include muscovite (clear), biotite (black), and phlogopite (dark brown). The different colors of mica can be explained by their large range of chemical compositions. Micas always contain silica and often contain elements such as potassium, sodium, or calcium (in one structural position) and aluminum, magnesium, or iron (in another structural position). More rarely, other elements can fill these structural positions, which can lead to some amazing color variations. For example, lepidolite contains lithium and can be purple or pink in color. As another example, fuchsite contains chromium and is bright green in color. Micas are soft minerals, generally about 2.5-3 on the Mohs hardness scale.
Mica is one of my favorite minerals. Who doesn’t love a shiny mineral that comes in a variety of fun colors?
The photograph above shows a large piece of muscovite that I have in my personal rock collection. I collected it from a pegmatite, a rock that commonly contains large crystals.
I recently saw some gorgeous specimens of mica when I visited the Museum of Mines and Metal in Belo Horizonte, Brazil. Below are some pictures of the mica specimens on display at the museum. Enjoy!





Geology Word of the Week: L is for Luster

def. Luster (or Lustre if you use British spelling):
The way in which the surface of a mineral or rock interacts with light. Words used by geologists to describe luster include metallic, sub-metallic, dull (or earthy), vitreous, waxy, silky, greasy, pearly, and adamantine.
Luster is a physical property that is used by geologists to help identify minerals and rocks. Other physical properties that geologists use for identification are hardness, density, magnetism, color, streak (the color of the mark a rock or mineral leaves on a streak plate), cleavage, and crystal form.
When using luster to identify a mineral, it is important to know that many minerals can have more than one type of luster, in the same way that many minerals come in different colors. Thus, luster should be used cautiously for mineral identification. A good example is shown in the photograph at the top of this post. This photograph shows a shiny rock with a metallic luster. At first glance, you might think that the rock contains mica, which can have a similar metallic luster and a similar color. However, the rock is actually an iron-rich rock that mostly consists of hematite. The portion of the rock with the metallic luster is specularite, also known as specular hematite. Note that a portion of the rock has a reddish brown color. This part of the rock has a dull or earthy luster and is also hematite. To properly identify the minerals in this rock, a geologist must use other properties in addition to luster. For example, the density of the rock (iron is heavy) and the streak of the rock. Specular hematite will leave a reddish brown streak while mica will leave a whiteish streak.
The photograph below shows another example of luster. In this case, there are quartz crystals with a vitreous (or glassy) luster.

The photograph below shows anther example of luster. This rock contains serpentine and talc. The white talc has a pearly or perhaps waxy luster. The green serpentine has a silky luster.

That’s all for this week’s word… stay tuned for next week!
Geology Word of the Week: K is for Karst

Several years ago, I used to write a “Geology Word of the Week” post in which I selected a word used by geologists, wrote a definition of the word, and wrote up a post with some information and pictures related to the word. I went through the alphabet in order twice, writing about words starting with letters from A to Z, and then I started a third run through the alphabet, stopping at my last post J is for Jasper back in 2015. Many people enjoyed my geology word posts, but eventually life became too busy for me to keep up the posts. Since 2015 I’ve obtained an M.Sc. degree in Carbon Management, moved countries, changed jobs twice, and had a child… all while working full-time during a global pandemic! Life is still very busy, but it isn’t quite as busy as it used to be.
For awhile, I’ve been thinking about reviving my weekly geology word posts… and I’ve decided that this year I will do so! I will do my best to post about a geology word every week. I may keep some of the posts a bit shorter than I used to, but I think they will still be informative.
Without further ado, here is the first word for 2023…
def. Karst:
A type of topography or landscape characterized by the dissolution of soluble rocks, such as limestone and dolomite. The dissolution of the rocks leads to the formation of landforms such as sinkholes, caves, and springs. Water is often stored underground in karst landscapes, and thus they can form aquifers that provide sources of water for humans and animals. The sinkholes that form in karst environments are geological hazards that must be monitored and managed.
Here is a great figure showing some typical features of a karst landscape:
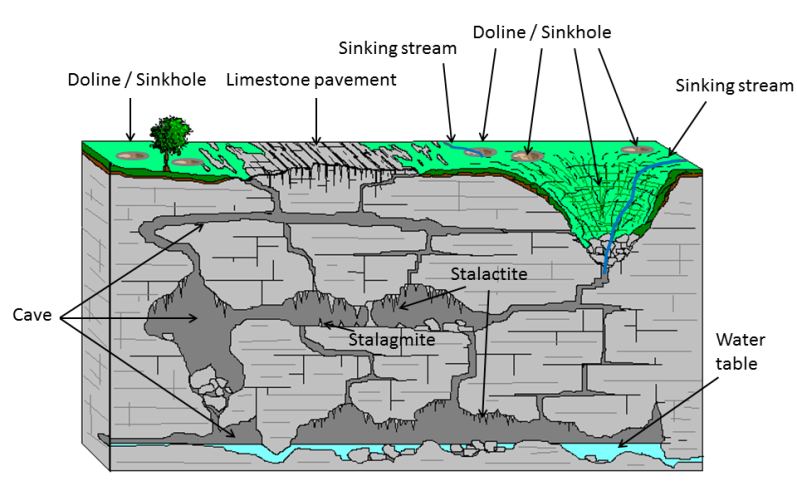
Most karst landscapes form in carbonate rocks, such as limestone or dolomite. In these rocks, the dissolution is caused by weak carbonic acid that forms when rainwater picks up carbon dioxide from the atmosphere and soil. In the open spaces of caves that form underground, the carbon dioxide in the water can be released, which causes the formation of speleothems, such as stalactites and stalagmites. If you’ve ever been in a cave with stalactites and stalagmites, then you’ve been in a karst landscape.
Below are a few pictures from Howe Caverns, a limestone cave in New York State that I recently visited with my family. You can visit an underground lake in this cave – and you can even take a boat ride on the lake! If you want to see a karst landscape up close, then I recommend a visit to Howe Caverns or another cave system.




That’s all for this week’s word. Stay tuned for next week!
What to Buy a Geologist for Christmas: 2022 Edition

This year, I’ve decided to post another list of great books to buy for the geologist in your life. All of these are available as e-books, so they make excellent last minute gifts.
If you want to look at previous years gift lists, here they are:
Book #1:
The Floor of Heaven: A True Tale of the Last Frontier and the Yukon Gold Rush
by, Howard Blum

I read this book several years ago when I was working as an exploration geologist in Alaska, and I really enjoyed it. The book is well written and captivates you with a thrilling – and true! – story from the Yukon Gold Rush.
Available from Amazon here. $13.99 on Kindle.
Book #2:
Earth: An Intimate History
by, Richard Fortey

This classic book by Richard Fortey is an excellent read. I can also recommend his books Life and Trilobite.
Available from Amazon here. $12.99 on Kindle.
Book #3:
A Portrait of the Scientist as a Young Woman
by, Lindy Elkins-Tanton

This is a delightful memoir by planetary scientist Lindy Elkins-Tanton. I was fortunate enough to spend some time with Lindy when we were both based at MIT. She is an amazing woman, and you will enjoy her book.
Available from Amazon here. $15.99 on Kindle.
Book #4:
Bright Galaxies, Dark Matter, and Beyond: The Life of Astronomer Vera Rubin
by, Ashley Jean Yeager

Here is another great book about an incredible scientist, the astronomer Vera Rubin. Highly recommended!
Available from Amazon here. $14.99 on Kindle.
Book #5:
How to Build a Habitable Planet: The Story of Earth from the Big Bang to Humankind
by, Charles Langmuir and Wallace Broecker

This classic book, written by two famous geologists, is a must-read for any geologist. I first read this book as a graduate student, and I learned so much about the formation and evolution of the planet.
Available from Amazon here. $34.99 on Kindle. There is also an older, free version of the book here.
Book #6:
The Dinosaur Hunters: A True Story of Scientific Rivalry and the Discovery of the Prehistoric World
by, Deborah Cadbury

This delightful book tells the story of the rivalry between two 19th century geologists: Gideon Mantell and Richard Owen. The book also tells much about 19th century fossil hunting and interpretation.
Available on Amazon here. $8.99 on Kindle.
Book #7:
Mapping the Deep: The Extraordinary Story of Ocean Science
by, Robert Kunzig

This is a fantastic, easy-to-read book on oceanography and marine geology. The writing is excellent. Very highly recommended.
Available from Amazon here. $13.77 on Kindle.
Book #8:
Missions to Mars: A New Era of Rover and Spacecraft Discovery on the Red Planet
by, Larry S. Crumpler

This is a great book about some of the recent Mars missions, written by a NASA scientist. A great read!
Available on Amazon here. $14.99 on Kindle.
Book #9:
The Red Planet: A Natural History of Mars
by, Simon Morden

This is another great book on Mars, written by a science fiction author (although this is, of course, non-fiction) with a Ph.D. in geophysics.
Available on Amazon here. $17.99 on Kindle.
Book #10:
The New Climate War: The Fight to Take Back Our Planet
by, Michael E. Mann

Last but not least, I can recommend this excellent book on climate change, which discusses the role that big fossil fuel companies have played in climate change misinformation. This book is alarming, but also inspires hope. Michael Mann convinces you that it is not too late to avert the worst of climate change.
Available from Amazon here. $12.99 on Kindle.
Happy holidays, everyone! I hope you have a relaxing, lovely time with your family and friends.









